Looking for oligos? Visit our new oligo and Stellaris dedicated platform at
oligos.biosearchtech.com

Rapid and simple purification kits for high-quality DNA from plants, livestock and cell cultures
Save time and streamline your nucleic acid purification workflow with sbeadex™ Lightning kits.
Powered by superparamagnetic bead technology, this revolutionary approach delivers exceptional purity and yield in as little as 5 minutes for a variety of lysed plant and animal sample materials and 11 minutes for cell cultures.
Our simple and convenient kits reduce plastic and liquid waste, cut shipping and storage costs, and offer scalable, automation-ready solutions.
For information about the specific sample type/respective kit, please click the icons below.
Why switch to Lightning?
- High-quality, high-yield DNA: Reliable performance for all downstream applications (e.g. PCR, targeted GBS, microarrays, NGS sequencing).
- Ultra-fast: DNA Purification in as little as 5 minutes from a wide range of samples including plant, livestock, and cell cultures.
- Scalable: Easily adapted to high-throughput automation. Compatible with most popular robotic platforms (e.g. KingFisher, Dynamic Devices Lynx, Hamilton, Tecan, Beckman-Coulter or our high-throughput oKtopure™ instrument)
- Cost-effective and sustainable: Reduced protocol steps cuts lab costs and reduces plastic, liquid, packaging and hazardous waste.
- High stability: Ready-to-use reagents with high stability at room temperature.
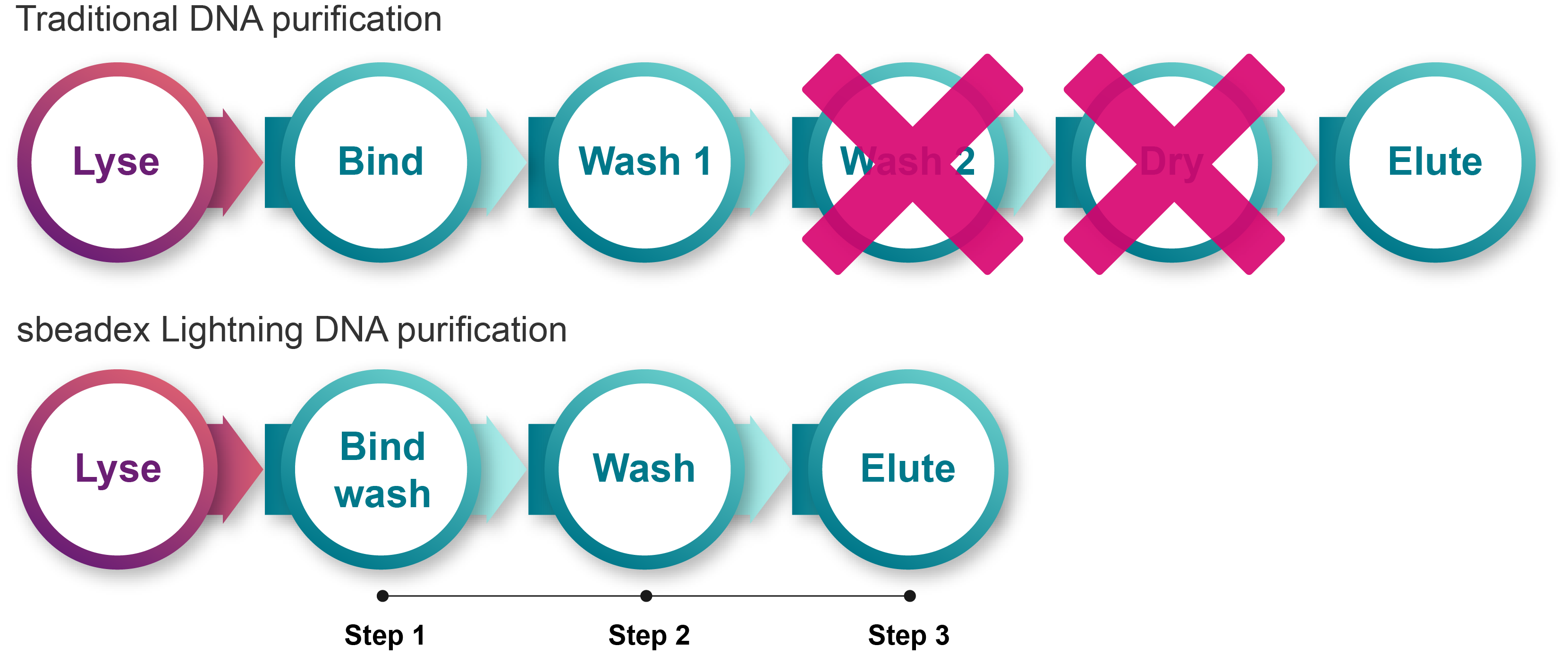
Transforming the traditional, time-consuming steps of nucleic acid purification – sbeadex Lightning revolutionises your workflow, reducing it to just 3 steps and 5 minutes.
How sbeadex Lightning works
Live demo of DNA purification in under 5 minutes
Powered by superparamagnetic particles and a novel binding chemistry enabling simultaneous DNA binding and washing, sbeadex™ Lightning delivers rapid, high-efficiency nucleic acid purification. The purification process is streamlined into a simplified 3-step protocol (excluding lysis) that can take as little as 5 minutes and eliminates the need for ethanol or chaotropic salt washes. A single water wash effectively removes impurities and potential inhibitors, preparing DNA for a wide range of downstream applications including PCR, RT-PCR, targeted genotyping by sequencing, NGS, microarrays, and restriction analysis. .

sbeadex Lightning Plant DNA Kits
Ultra-fast DNA purification from plant leaves and seeds – taking you from lysate to eluate in just 5 minutes
The sbeadex Lightning plant kit is available in two formats:
-
Standard version:
sbeadex Lightning Plant DNA Kit
Designed for manual or automated DNA extraction from up to 100 mg of fresh plant material. Suitable for use in tubes or 96-well plates. -
HTP (high-throughput): sbeadex Lightning Plant HTP DNA Kit
A miniaturised format optimised for 96-well or 384-well plate processing, using reduced reaction volumes for high-throughput applications.
| sbeadex Lightning Plant DNA Kit | sbeadex Lightning Plant HTP DNA Kit | |
|---|---|---|
| Format | Off-the-shelf catalogue kit | Off-the-shelf catalogue kit |
| Technology | sbeadex magbeads with Lightning binding chemistry | sbeadex magbeads with Lightning binding chemistry |
| Sample Type | Leaves, seeds | Leaves, seeds |
| Sample input | Up to 100 mg fresh or 30 mg dry | Up to 30 mg fresh or 5 mg dry |
| Processing format | Single tubes or 96-well | 96- or 384-well |
| Protocol time | Down to 5 mins excl. lysis | Down to 5 mins excl. lysis |
| Product formats and catalogue numbers |
96 reactions: NAP40-035-01 960 reactions: NAP40-035-02 10,000 reactions: NAP40-035-03 |
1,536 reactions: NAP40-036-01 10,000 reactions: NAP40-065-02 |
High quality sample collection and preparation can help to produce the best results in your plant genotyping workflow. Read our blog for information about the best practices for sample processing for NGS projects, or tips about the complexity of your sample composition.
Need a simple and efficient sample collection kit? Try BioArk Leaf or BioArk Seed.
Our sbeadex Lightning purification chemistry has been successfully tested on a wide range of plant samples and delivers high-quality DNA in a fraction of the time required by conventional magnetic bead-based kits.
| Type | Tissue | Species |
|---|---|---|
| Plant | Fruit | Blackberry, Blueberry, Potato |
| Leaf | Apple, Banana, Blackberry, Brassica, Cabbage, Cannabis, Canola, Canola, Carrot, Cocoa, Corn, Cucumber, Currant, Hop, Melon, Oat, Onion, Papaya, Parsley, Pepper, Potato, Rice, Soy, Strawberry, Sugar Beet, Sunflower, Tomato, Wheat | |
| Seed | Barley, Canola, Corn, Cucumber, Lentil, Oat, Pea, Rice, Sorghum, Soy, Sugar Beet, Sunflower, Tomato, Wheat |

sbeadex Lightning Livestock DNA Kits
Save valuable time in your livestock breeding and DNA testing programmes with our
3-step/5-minute protocol
The sbeadex™ Lightning Livestock DNA Kit helps breeders tackle bottlenecks in sample processing
by reducing reagent costs and enabling scalable automation across diverse livestock sample types
- all within one unified purification workflow.
Unlike traditional solutions that require separate kits for
tissue, TSUs, hair, or swabs, Lightning combines optimised lysis protocols into a single streamlined process that feeds into a rapid, automation-ready purification.
This approach delivers high-quality DNA for PCR, microarrays, and NGS applications such as KASP™, Amp-Seq One, and Flex-Seq, ensuring greater efficiency and faster turnaround times.
Our sbeadex Lightning purification chemistry has been successfully tested on a wide range of animal samples and delivers high-quality DNA in a fraction of the time required by conventional magnetic bead-based kits.
| Livestock | Blood (in storage buffer) | Chicken |
| Dry swabs | ||
| Ear punch (TSU) | Bovine, Mouse, Pig | |
| Fin tissue | Salmon, Trout | |
| Genotek swabs | ||
| Hair | Bovine, Horse | |
| Muscle | Bovine, Trout, Chicken | |
| Tail clip | Mouse |
High quality sample collection and preparation can help to produce the best results in your livestock genotyping workflow. Read our blog to learn 4 steps to optimise sample collection for aquaculture genotyping.
Need a simple and efficient sample collection kit for fish fins? Try BioArk Fish.

sbeadex Lightning Cell DNA Kit
Rapid and simple DNA purification for cell culture applications
The sbeadex Lightning Cell DNA Kit is effective for a variety of cell types, including HeLa, HEK293 and CHO cell cultures and peripheral blood mononuclear cells (PBMCs). The kit is suitable for DNA isolation from ≤1x106 cells per reaction.
Ideal for workflows that require screening large numbers of cell culture samples, such as quality control of gene editing events.
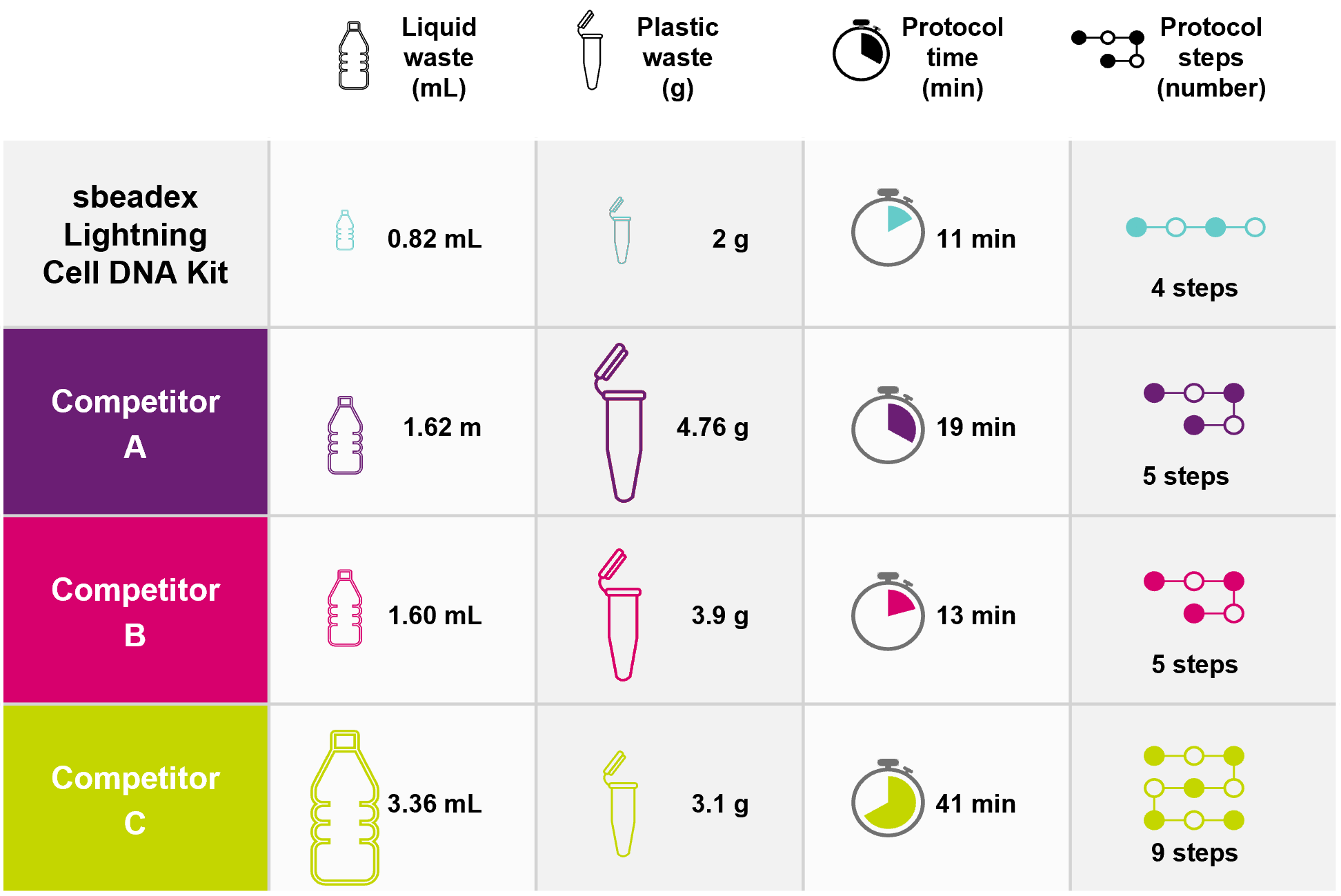
Summary of the key savings for the sbeadex Lightning Cell DNA Kit compared to market-leading competitors.
This schematic illustrates the savings in protocol time, protocol steps, plastic consumables and liquid waste per sample. Values are based on processing HEK293 cells. Protocol time per sample (minutes) refers to incubation times only and includes cell lysis. Number of protocol steps includes lysis. Liquid waste (mL) excludes elution volume.

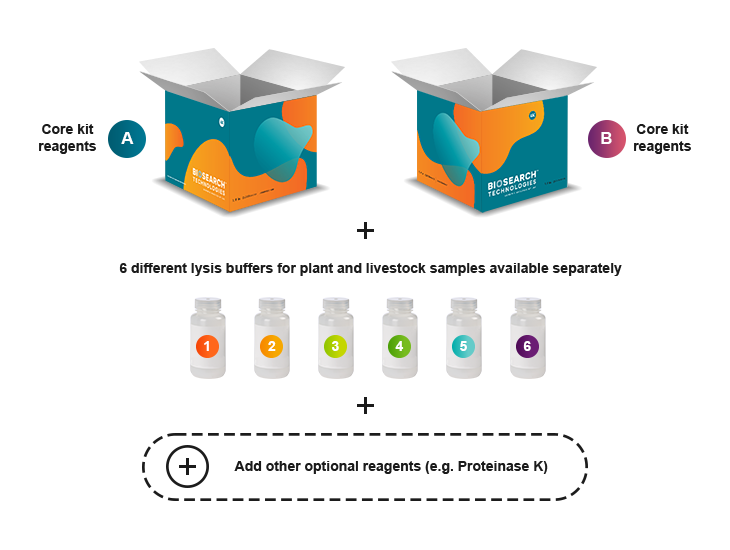
sbeadex Lightning modular kits
Tailor our Lightning Chemistry and Lysis to your needs.
What is a modular DNA Kit and how does it work?
Our Modular DNA Kit is designed to give you maximum flexibility in DNA extraction workflows. Instead of a one-size-fits-all solution, you can custom-build your kit to match your sample type, throughput needs, and automation setup.
Using a modular approach, you combine:
- Lysis Buffers: Choose from six different chemistries tailored to various sample types.
-
Purification: Our sbeadex Lightning Core Kits — select either Kit A or Kit B, each containing:
- Binder Buffer
- sbeadex particle suspension
- Elution Buffer
- Optional Add-ons: Enhance your workflow with reagents like Proteinase K, RNase, or Debris Capture Beads.
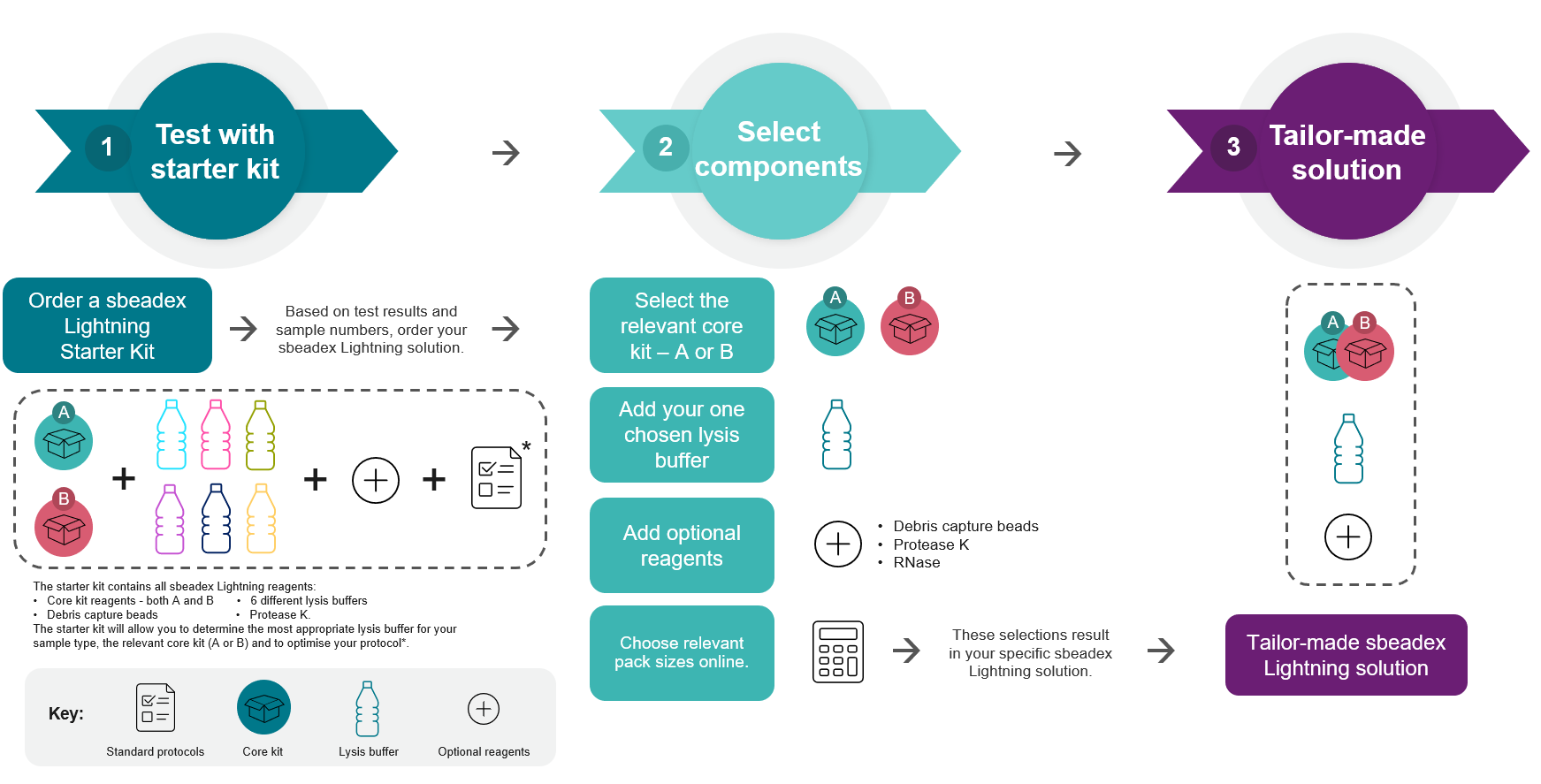
How to get started and choose the right components?
To help you find the best configuration, we
offer Starter Kits that include all components needed for
optimisation. These kits allow you to:
- Test different lysis buffers and purification options
- Evaluate add-ons for your specific sample type
- Fine-tune your workflow for performance and efficiency
Once you've identified the ideal setup, you can order only the components you need – saving cost and reducing waste.

Figure 1. How to get started with sbeadex Lightning
technology.
1. Order a sbeadex Lightning Starter Kit and test this with your
samples. Once you have optimised your protocol, (2.)
select/order the
components to generate your tailor-made sbeadex Lightning
solution (3.).
* Please find standard protocols and sample-specific lysis buffer
recommendations online or contact
techsupport@lgcgroup.com
for assistance.
| Plant tissue | ||
|---|---|---|
| leaf | ||
| cucumber leaves | barley leaves | carrot leaves |
| brassica leaves | corn leaves | melon leaves |
| tomato leaves | potato leaves | cannabis leaves |
| pepper leaves | pea leaves | oat leaves |
| wheat leaves | parsley | |
| seed | ||
| corn seed | canola seed | soy seed |
| sunflower seed | ||
| fruit | ||
| blackberry | blueberry | potato |
| Animal tissue | |
|---|---|
| bovine ear punch | mouse ears |
| bovine meat | mouse tail |
| bovine hairs | Chicken (wing) |
| pig ear punches | Salmon (fin) |
| Trout (muscle biopsy) | Beef (muscle tissue biopsy) |
| Biological fluids | |
| chicken blood (in storage buffer) | |
Table 2: Animal sample types successfully tested using the sbeadex Lightning chemistry for DNA purification.
Can’t see your sample type here? Get in touch
Looking for a custom solution?
Our R&D team can develop a solution specifically for your project. Get in contact with us below

sbeadex Lightning supports your sustainability goals by reducing plastic use by 50% and liquid waste by 60% on average, while also cutting energy consumption for shipping, storage and instrumentation – reducing CO2 emissions and cost too.
This fast, simple and convenient workflow is more environmentally friendly than other comparable kits in the market.
Need support?
Please reach out if you would like help placing your order.
| Europe, Middle East, and Africa | |
|---|---|
| UK | +44 1992 470 757 |
| Germany | +49 30 1663 54600 |
| North America, Latin America | |
| Wisconsin, USA | +1 888 575 9695 |
| Asia Pacific | |
| China | +8621-22509000 |
| Singapore | +65 6734 4800 |