Looking for oligos? Visit our new oligo and Stellaris dedicated platform at
oligos.biosearchtech.com

Discover the difference of sbeadex technology
Experience sbeadex nucleic acid purification kits, where advanced technology meets seamless user-friendly operation. All of our sbeadex nucleic acid purification kits leverage the power of magnetic bead technology by utilising superparamagnetic particles coated with sbeadex surface chemistry. A novel binding mechanism ensures high-quality DNA or RNA, removing impurities from your sample matrix, and preparing it for PCR, sequencing and other downstream applications. Our kits are optimised for high-throughput automation and are compatible with popular robotic platforms including KingFisher, Hamilton, Tecan, Beckman-Coulter, and our fully automated nucleic acid extraction platform oKtopure™.
Tell me more about sbeadex technologyExplore our sbeadex nucleic acid purification kits

Experience the evolution of nucleic acid purification
- High purity: Elevate your research with nucleic acids of exceptional purity, meticulously isolated using our advanced sbeadex magnetic bead technology.
- Streamlined protocols: Experience efficiency in every step. Our kits offer fast, convenient, and reproducible protocols that seamlessly integrate into high-throughput automation, saving you time and effort.
- Versatile applications: From basic and biopharmaceutical research to molecular diagnostics, our sbeadex technology is applicable across a diverse range of sample types, ensuring adaptability to your specific research needs.
- Inhibitor-free: Bid farewell to potential PCR inhibitors. Our water-based wash buffers are meticulously designed to eliminate any hindrance to your downstream applications, providing nucleic acids ready for various assays.
- Stability and compatibility: Trust in the high stability of our ready-to-use kits, compatible with popular robotic platforms such as KingFisher, Hamilton, Tecan, Beckman-Coulter or our high-throughput oKtopure instrument. Your research deserves reliable tools, and we deliver.
Harness the power of sbeadex technology
Quality is in our DNA. Our mission is to deliver the highest quality products and services and consistently exceed customer expectations. We continue to make investments to ensure that our design, manufacturing and shipping processes comply with industry standards.
In contrast to traditional methods, our technology streamlines the process by eliminating the need for a drying step before elution, ensuring exceptional DNA quality. Prioritising sustainability, our kits feature an ethanol-free second wash buffer which helps to remove impurities that might inhibit enzymatic downstream reactions.
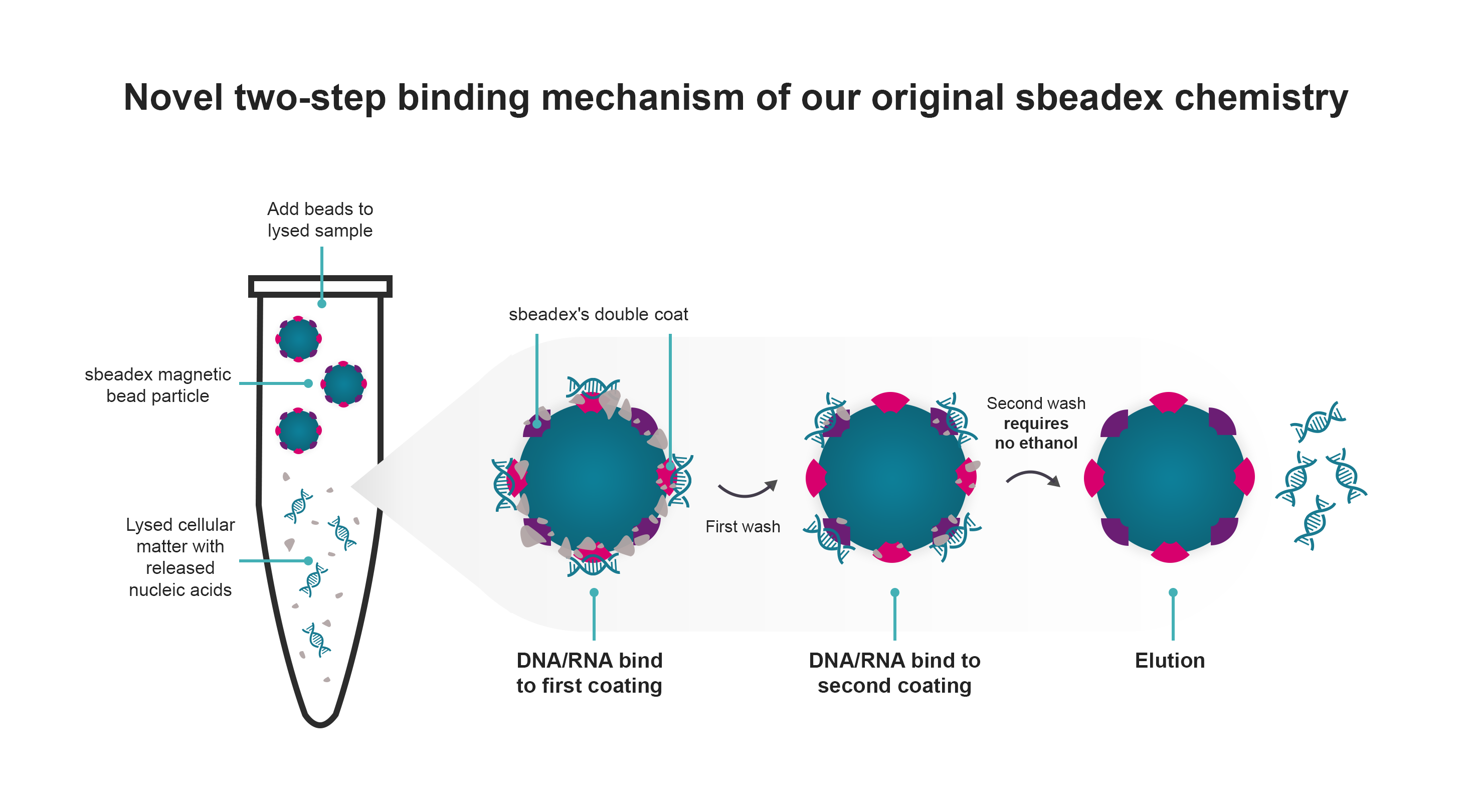
Versatility is at the core of our technology, making it suitable for diverse applications, from basic biopharmaceutical research to molecular diagnostics, and agricultural screening. The adaptable and reproducible protocols, along with compatibility with popular robotic platforms, position sbeadex kits as a reliable solution for high-throughput automation.
Efficient DNA/RNA preparation with sbeadex magnetic beads: streamlined protocol for high-quality nucleic acids
Discover a rapid and efficient DNA/RNA preparation protocol using sbeadex magnetic beads. Achieve high-quality purification of small and large DNA and RNA fragments within just 45 minutes, eliminating the need for centrifugation steps. Our double-coated beads employ a unique dual binding protocol, enabling the elution of ultrapure nucleic acids directly into water. Experience reduced solvent carryover, ensuring the integrity of your samples for optimal downstream applications

sbeadex Mini and Maxi Plant DNA Purification Kits
Providing a comprehensive solution, the sbeadex Mini and Maxi Plant DNA Purification Kits include all the necessary components for both small and large-scale DNA purification from plant tissue. They have been optimised for genomic DNA isolation from both fresh and lyophilised plant tissue material including leaves, seeds, fruits, pulp, bark and roots.
We offer five plant kits, starting with the mini plant trial kit that has been designed to evaluate and optimise performance workflow. Our standard mini plant kit, as well as the mini plant kit without dangerous goods to ensure safety and regulatory compliance, are tailored for small-scale purification of high-quality plant DNA.
Additionally, we offer the standard maxi plant kit and the maxi plant kit without dangerous goods, both designed for large-scale plant DNA purification.
sbeadex plant kits have been successfully used with:
| Plant species | Leaves | Seed |
|---|---|---|
| Apricot (Prunus armeniaca) | X | |
| Barley (Hordeum vulgare) | X | X |
| Beet, sugar (Beta vulgaris) | X | |
| Canola/oilseed (Brassica napus) | X | X |
| Chicory (Cichorium intybus) | X | |
| Corn (Zea mays) | X | X |
| Cotton (Gossypium) | X | X |
| Cucumber (Cucumis sativus) | X | X |
| Flax (Linum usitatissimum) | X | |
| Grape (Vitis vinifera) | X | X |
| Lettuce (Lactuca sativa) | X | |
| Muskmelon (Cucumis melo) | X | X |
| Onion (Allium cepa) | X | X |
| Parsley (Petroselinum crispum) | X | X |
| Peach (Prunus persica) | X | |
| Pepper (Capsicum annuum) | X | X |
| Potato (Solanum tuberosum) | X | |
| Rice, Asian (Oryza sativa) | X | X |
| Rubber (Hevea brasiliensis) | X | X |
| Soybean (Aphis glycines) | X | X |
| Sunflower (Helianthus annuus) | X | X |
| Tobacco leaves (Nicotiana tabacum) | X | X |
| Tomato (Solanum lycopersicum) | X | X |
| Wheat (Triticum L.) | X | X |
Typical quantities of starting material are:
- 5-10 mg lyophilised tissue or 10-30 mg fresh tissue for the sbeadex Mini Plant DNA Purification Kit
- 10-30 mg lyophilised tissue or 40-120 mg fresh tissue for the sbeadex Maxi Plant DNA Purification Kit
| Title | Type |
|---|---|
| General sbeadex Plant DNA Purification Kit manual (valid for sbeadex Mini and Maxi kits) | Manual |
| sbeadex Mini Plant DNA Purification Kit (trial kit) | Protocol |
| sbeadex Mini Plant DNA Purification Kit | Protocol |
| sbeadex Maxi Plant DNA Purification Kit | Protocol |
| sbeadex Plant DNA Purification Kits | Data sheet |
| sbeadex plant kit application note (for seed) | Application note |
| The oKtopure™ and sbeadex™ plant nucleic acid extraction kit | Application note |
| sbeadex maxi plant kit and KingFisher 96 instrument | Application note |
| sbeadex mini plant kit and KingFisher 96 instrument | Application note |
| oKtopure automated nucleic acid purification | Data sheet |
| sbeadex plant DNA purification safety data sheets | SDS |
sbeadex Pathogen Nucleic Acid Purification Kits
Offering a cutting-edge solution, the sbeadex Pathogen Nucleic Acid Purification Kits ensure fast and reliable isolation of highly purified DNA and RNA from various pathogenic bacteria, viruses and yeasts. We provide four specialised kits to cater to your research needs.
Options include our kit with Proteinase K to boost extraction efficiency by aiding in cell disruption and nucleic acid release, a version without Proteinase K, one without dangerous goods, and another without both Proteinase K and dangerous goods.
All of our sbeadex Pathogen Nucleic Acid Purification kits are user-friendly kits designed to streamline the purification process of pathogenic DNA and RNA, making them an excellent choice for downstream molecular diagnostic applications. The purification procedure can be fully automated and is compatible with many robotic platforms.

The sbeadex Pathogen testing kits have been used successfully with:
| sbeadex Pathogen Nucleic Acid Purification Kit | Competitor A | ||||
| N | Average Cq | StDev | Average Cq | StDev | |
| Clean up sample | 12 | 30.35 | 0.14 | 33.80 | 0.15 |
| Direct spike-in sample | 3 | 31.00 | 0.44 | 33.80 | 0.12 |
Figure 1: Demonstration of no loss of RNA during the sbeadex Pathogen Nucleic Acid Purification Kit protocol. 500 copies of a 2019-COVID artificial RNA reference material were added to a sample (nasopharyngeal swab) and the sample processed with the sbeadex Pathogen Nucleic Acid Purification Kit. SARS-CoV-2 was detected in this preparation and a directly spiked positive control sample via a RT-qPCR at two separate Biosearch Technologies sites. The concordant Cq values from both samples demonstrate no loss of sample due to the extraction step
| Pathogen | External Control (Artificial Matrix) |
| Viral Targets | |
| SARS-CoV-2 | VTM1 |
| Influenza A H3N2 | Swab2 |
| Influenza B | Swab2 |
| Novel Influenza A H1N1 | Swab2 |
| Respiratory Syncytial Virus (subtype A) | Swab2 |
| Respiratory Syncytial Virus (subtype B) | Swab2 |
| Adenovirus 4 | Swab2 |
| Zika Virus | Plasma3 |
| Cytomegalovirus | Plasma4 |
| Human Papillomavirus-16 | Methanol-preserved cultured human cells containing full-length HPV episomal DNA5 |
| Bacterial Targets | |
| Bordetella pertussis | Swab5 |
| Chlamydophila pneumoniae | Swab5 |
| Chlamydophila psittaci | Swab5 |
| Coxiella burnetii | Swab5 |
| Legionella pneumophila | Swab5 |
| Mycoplasma pneumoniae | Swab5 |
| Mycobacterium tuberculosis | Sputum7 |
| Campylobacter jejuni | Stool8 |
| Salmonella enteritidis | Stool8 |
| Yersinia enterocolitica | Stool8 |
| Clostridium difficile | Stool8 |
| Chlamydia trachomatis | Urine9 |
| Neisseria gonorrhoeae | Urine9 |
| Staphylococcus aureus | External Run Control10 |
| Other Targets | |
| Candida albicans | Positive Extraction Control11 |
| Compatible Matrices Tested | Swabs (UTM®/VTM) Sputum Saliva Blood Plasma Serum Urine Stool Cerebrospinal Fluid |
Table 1: Pathogens that have already been tested using the sbeadex Pathogen Nucleic Acid Purification Kit.
Standards used for testing
- AccuPlex™ SARS-CoV-2 Verification Panel - Full Genome, 0505-0168 (LGC SeraCare)
- AMPLIRUN® TOTAL RESPIRATORY VIRAL PANEL CONTROL (SWAB), MBTC020 (Vircell S.L.)
- AMPLIRUN TOTAL ZIKV/DENV/CHIKV CONTROL (PLASMA), MBTC023 (Vircell S.L.)
- AMPLIRUN TOTAL CMV CONTROL (PLASMA), MBTC018-R (Vircell S.L.)
- AccuTrak™ HPV Genotype Qualification Panel, QSH701 (2400-0161), (LGC SeraCare)
- AMPLIRUN TOTAL ATYPICAL BACTERIAL PNEUMONIA CONTROL (SWAB) MBTC022-R (Vircell S.L.)
- AMPLIRUN TOTAL MTB CONTROL (SPUTUM) MBTC013 (Vircell S.L.)
- AMPLIRUN TOTAL GASTROINTESTINAL BACTERIAL PANEL CONTROL (STOOL), MBTC021 (Vircell S.L.)
- AMPLIRUN TOTAL CT/NG CONTROL (URINE), MBTC003 (Vircell S.L.)
- NATtrol™ S. aureus (MRSA) External Run Control, NATSAU(MRSA)-ERCM (Zeptometrix Corporation)
- NATtrol Candida/TV Positive Control, NATCTVPOS-BD (Zeptometrix Corporation)
Note: Other sample matrices may also be used with this kit. Further testing on other pathogens and matrices is in progress and this table will be updated accordingly.

sbeadex Livestock DNA Purification Kits
Our universal solution for DNA purification, this kit serves as a comprehensive livestock DNA purification solution that requires only one kit for different livestock samples.
With its universal approach designed to streamline workflows, it provides DNA purification suitable for a wide range of downstream applications. The sbeadex Livestock DNA Purification Kit contains all of the components necessary to perform high-quality DNA purification. We offer our standard livestock DNA purification kit as well as a kit version that does not contain dangerous goods.
Types of applications include nucleic acid isolation from a variety of samples such as tissue, blood, hair, semen or saliva from different livestock (e.g. bovine, chicken, fish, dog, horse and sheep) - for genetic screening and livestock DNA testing in animal breeding as well as detection of genetic diseases in animals.
The sbeadex livestock DNA testing kits have been used successfully with:
| Sample type | Animal species | |
|
|
|
| Title | Type |
|---|---|
| sbeadex Livestock DNA Purification Kit | Manual |
| sbeadex Livestock DNA Purification Kit | Datasheet |
| oKtopure automated nucleic acid purification | Data sheet |
| oKtopure tip wash station | Technical note |
| Genotyping solutions | Flyer |
| sbeadex livestock DNA purification safety data sheets | SDS |
sbeadex Forensic DNA Purification Kits
Purify DNA from a range of forensic material such as buccal swabs, hair roots, fingernails, whole blood and blood stains, saliva, tissue and semen. The sbeadex Forensic DNA Purification Kit stands out as a highly efficient magnetic bead-based solution. Easily modify batch size, lysis steps, and elution steps according to your requirements
We offer two kits tailored to meet your research requirements, a standard kit and a version without dangerous goods to ensure safety and regulatory compliance. Both kits are compatible with a variety of commonly used forensic sample types, ensuring the purity of the forensic DNA sample for subsequent processing.

| Title | Type |
|---|---|
| sbeadex Forensic DNA Purification Kit | Manual |
| sbeadex Forensic DNA Purification Kit | Data sheet |
| sbeadex Forensic DNA Purification Kit for automated processing | Application note |
| sbeadex Forensic PCR clean up | Application note |
| Sensitivity of the sbeadex Forensic DNA Purification Kit | Application note |
| Validation of custom sbeadex Forensic DNA Purification Kit | Application note |
| Comparison of the sbeadex Forensic DNA Purification Kit | Application note |
| sbeadex Forensic DNA Purification Kit and the KingFisher 96 instrument | Application note |
| sbeadex forensic DNA purification safety data sheets | SDS |
sbeadex Blood DNA Purification Kits
Streamline your blood DNA purification workflow with the sbeadex Blood DNA Purification Kit. Using advanced magnetic bead technology, this kit excels in handling human blood samples.
Overcome the intricacies of blood samples with this standardised and robust nucleic acid purification solution. Resulting in ready-to-use samples, this kit is tailored for downstream applications, from genotyping to next generation sequencing (NGS). We offer our standard blood DNA purification kit as well as a kit version that does not contain dangerous goods to ensure safety and regulatory compliance.
| Title | Type |
|---|---|
| sbeadex Blood DNA Purification Kit | Manual |
| sbeadex Blood DNA Purification Kit | Data sheet |
| sbeadex Blood DNA Purification Kit and anticoagulant preservatives | Application note |
| Automation of the sbeadex Blood DNA Purification Kit using Hamilton robotics | Application note |
| sbeadex blood DNA purification safety data sheets | SDS |
sbeadex Plasmid DNA Purification Kit
Streamline your plasmid DNA extraction with our sbeadex Plasmid DNA Purification Kit. This kit efficiently purifies high-quality plasmid DNA, specifically optimised for E. coli strains commonly used in labs.
Our kit serves as a versatile tool for various applications, including cloning, restriction analysis, in vitro transcription, cell transformation/transfection, nucleic acid labelling, PCR, and DNA sequencing. Developed and optimised to obtain good yields of highly pure plasmids from smaller volumes of bacterial culture (1 mL), this kit provides a reliable foundation for advanced molecular biology.

sbeadex Tissue DNA Purification Kit
Unlock the potential of your research with the sbeadex Tissue DNA Purification Kit, designed to extract high-quality DNA from a diverse range of human and animal tissue materials.
Experience efficient DNA purification in just one hour, preparing high-quality DNA for seamless downstream processing. Compatible with a diverse range of sample types from skin and lung to muscle, spleen, mouse tails, and ear punches, this kit leverages magnetic bead-based technology, ensuring a streamlined and efficient DNA purification process for reliable and consistent results.
sbeadex Tissue DNA Purification Kits have been successfully used with:
- Mouse tails
- Mouse lung
- Ear punches
- Skin
- Spleen
- Muscle
sbeadex PCR Clean-Up Kit
Efficiently remove nucleic acid contaminants during a standard PCR with our PCR Clean Up Kit. Components of the polymerase chain reaction such that primers, unincorporated nucleotides, enzymes and other contaminations are efficiently removed during the purification process due to the chemical specificity and physical size of our sbeadex particles.
Ensure the purity of your PCR products in sensitive downstream applications with our PCR Clean Up Kit. Highly purified PCR products within 1 hour with minimal set-up time and ready-to-use buffers. Driven by magnetic bead technology, this kit efficiently eliminates post PCR contaminants, resulting in PCR products with superior quality.

| Title | Type |
|---|---|
| sbeadex PCR clean-up Kit | Manual |
| sbeadex forensic PCR clean-up | Application note |
| sbeadex forensic PCR clean-up safety data sheets | SDS |
Explore our diverse selection of sbeadex kits
View all sbeadex products View our extraction and purification brochureNeed support?
Please reach out if you would like help placing your order.
| Europe, Middle East, and Africa | |
|---|---|
| UK | +44 1992 470 757 |
| Germany | +49 30 1663 54600 |
| North America, Latin America | |
| Wisconsin, USA | +1 888 575 9695 |
| Asia Pacific | |
| China | +8621-22509000 |
| Singapore | +65 6734 4800 |