{{faqs.Name}}
-
{{category.Name}}:
-
| Catalog # | Price | Size/Scale | Note | Qty | Add | |
|---|---|---|---|---|---|---|
| {{item.catalogId}} | {{ item.userPriceBook.unitPrice | currency }} | {{ item.unitPrice | currency }} | {{item.sizeAndScale}} | {{item.note}} |
 {{item.message}}
{{item.message}}
|
|
| checkout view cart | ||||||
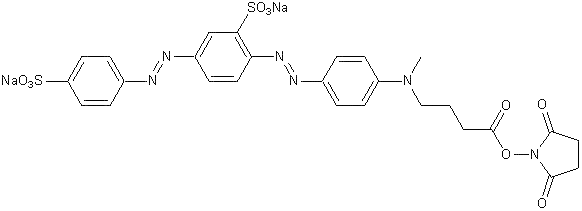
C27H24N6Na2O10S2
702.63
dark red solid
516
28700
Spectral properties measured in phosphate-buffered saline (PBS) pH 7.0.
Cold
-15 to -30 °C
BHQ®-10 Succinimidyl Ester is a water soluble dark quencher that can be coupled to nucleophilic groups such as the amines on proteins and peptides. It has been formulated to provide water solubility and to reduce non-specific protein binding interactions with proteins. As a result of Förster Resonance Energy Transfer (FRET), BHQ-10 is expected to quench fluorophores that have emission maxima close to 516 nm, such as fluorescein (FITC is fluorescein isothiocyanate).

Spectral properties measured in phosphate-buffered saline (PBS) pH 7.0.
Method for conjugation of BHQ10-OSu to proteins and antibodies: • BHQ-10 OSu is NOT COMPATIBLE with tris buffers • Use carbonate-bicarbonate, borate or phosphate buffer, pH 7.5 to 9.0, for conjugation of BHQ-10 OSu to proteins. • Prepare a stock solution of BHQ 10-OSu in DMF or DMSO and immediately add to the aqueous solution of protein (1-2 mg/mL) such that the organic solvent is no more than 10% of the final volume. Alternatively, add the solid BHQ-10 OSu to the protein solution and vortex or sonicate to ensure complete dissolution. • Separate the labeled protein from excess dye by size-exclusion chromatography, e.g. Sephadex G50, or by dialysis. Do not store stock solutions of this product for longer than two days. Product Stability: Reducing agents that are commonly used to reduce dithiol bonds in proteins, such as DTT and TCEP, will also reduce BHQ-10 thereby causing a loss of color and of quenching efficiency. Conjugation of BHQ-10 OSu to substrates in aqueous media: Amine reactive reagents such as succinimide esters react with non-protonated aliphatic amino groups, including the amine terminus of proteins and the epsilon-amino group of lysines. To maintain the amine groups in the non-protonated form, the conjugation reaction must take place in a buffer with slightly basic pH. More specific labeling of the amine terminus may be achieved by using a buffer closer to neutral pH, as the pKa of the terminal amino group is lower than that of the lysine epsilon amino groups. Buffers, such as Tris, that contain primary amines should not be used with this product. Instead borate, phosphate or carbonate buffers should be used. For best results with this product, we recommend using the buffers specified in the protocol below. The procedure may be scaled up or down, maintaining the same molar ratios of reagents. It is important to consider that the number and surface positions of the amino groups will vary greatly among proteins. The reactivity of the amino groups on proteins will vary according to the structure of the protein. If possible, we recommend experimentation with different molar ratios of reactive dye to protein in order to determine the protocol that yields the desired level of incorporation into the protein.