{{faqs.Name}}
-
{{category.Name}}:
-
| Catalog # | Price | Size/Scale | Note | Qty | Add | |
|---|---|---|---|---|---|---|
| {{item.catalogId}} | {{ item.userPriceBook.unitPrice | currency }} | {{ item.unitPrice | currency }} | {{item.sizeAndScale}} | {{item.note}} |
 {{item.message}}
{{item.message}}
|
|
| checkout view cart | ||||||
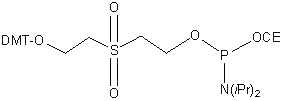
2-[2-(4,4'-Dimethoxytrityloxy)ethylsulfonyl]ethyl-(2-cyanoethyl)-(N,N-diisopropyl)-phosphoramidite
C34H45N2O5PS
656.77
colorless oil
Prior to dilution ensure that all product is at the bottom of the vial. Dilute to the recommended concentration and mix thoroughly in the sealed vial to ensure that all contents are dissolved.
100 µmol/mL
2-minute coupling
The deprotecting conditions for this amidite will depend on the types of amidites used for the synthesis of the oligo. If using fast deprotecting amidites, deprotect in concentrated NH4OH for 1 hour at 60 °C. If using standard amidites deprotect in concentrated NH4OH for 5 hours at 60 °C.
79.98
Cold
-15 to -30 °C
5' Phosphate Amidite enables the introduction of a phosphate group to the 5' terminus of an oligonucleotide. Oligonucleotides having a 5'-phosphate group are valuable tools for many molecular biology applications including gene construction, cloning, mutagensis, and ligation chain reaction. Quantification of the DMT protecting group removed during the standard synthesis deblocking step will yield phosphorylation efficiency.

The DMT group and the sulfonylethyl group will be eliminated during ammonia deprotection, thereby eliminating the option of DMT-ON cartridge purification. Purification: The modified 5’ phosphate oligonucleotide may be desalted using a Micropure purification cartridge or by ethanol preciptation. Purification of this product may be accomplished using gel electrophoresis or Ion exchange HPLC.

79.98